Targeted Lipid Nanoparticles in Nucleic Acid Delivery
Introduction
The advancement of lipid nanoparticles (LNPs) has revolutionized nucleic acid delivery, paving the way for innovative therapies across multiple disease areas. Targeted lipid nanoparticles (tLNPs) take this technology further—enhancing the precision of delivery by directing therapeutic agents like mRNA and siRNA to specific tissues or cell types. This targeted approach not only increases efficacy but also minimizes off-target effects, improving patient safety and outcomes. Most notably, LNPs gained widespread recognition for their role in COVID-19 mRNA vaccines. This blog explores how tLNPs are designed, how they work, and where they are headed in the future of drug delivery and gene therapy.
What are targeted-lipid nanoparticles (tLNPs)?
Lipid nanoparticles are versatile drug delivery vehicles composed of lipids such as cholesterol, helper lipids, PEGylated lipids (PEG-lipids), and ionizable lipids. These components work together to encapsulate and protect fragile nucleic acid cargo (like mRNA or siRNA), promoting cellular uptake and stability.
The "targeted" functionality in tLNPs is achieved by attaching ligands or antibodies to the nanoparticle surface. These molecules bind to specific receptors on target cells, directing the LNPs to the desired site of action. tLNPs are now considered the most efficient non-viral nucleic acid delivery systems, offering scalable, biocompatible solutions for gene expression and silencing.
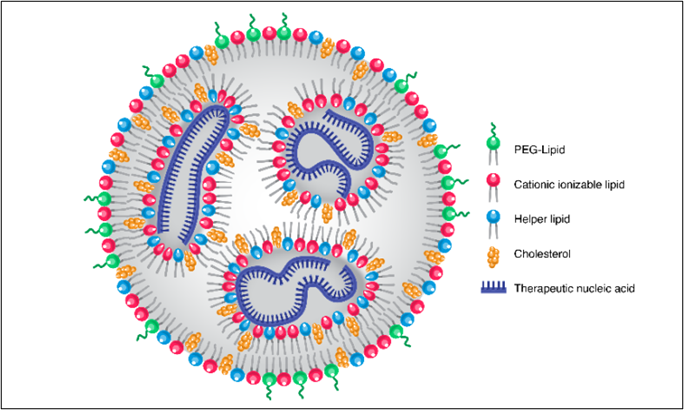
Figure 1: Structure of Lipid Nanoparticle
The evolution of lipid nanoparticles
The journey began with the direct injection of naked mRNA, which faced major limitations in stability and delivery efficiency. The development of LNPs addressed these barriers by enabling intracellular delivery and protecting nucleic acids from degradation.
Today, LNPs have evolved into FDA-approved delivery platforms for vaccines and rare disease treatments - most notably in COVID-19 vaccines and therapies like Onpattro (patisiran) for hereditary transthyretin amyloidosis.
How LNPs are made
LNPs are typically formulated using a method called alcohol dilution, where lipids in ethanol are rapidly mixed with nucleic acids in an aqueous buffer - often through microfluidic mixing. This process creates uniform nanoparticles with a high encapsulation efficiency.
To achieve targeted delivery, additional steps are taken, such as the inclusion of targeting ligands or modifying the lipid composition to enhance tissue specificity.
Targeting strategies: passive vs. active targeting
Passive targeting
Passive targeting relies on nanoparticle properties like size, surface charge, and hydrophilicity to direct where the LNPs accumulate. For example:
- Lymph node targeting: Small, negatively charged LNPs (~30–50 nm) are taken up more effectively by dendritic cells, making them ideal for vaccine delivery.
- Tumor targeting: Exploiting the enhanced permeability and retention (EPR) effect, LNPs preferentially accumulate in tumor tissue due to leaky vasculature.
Tuning the PEG-lipid content (e.g., increasing DMG-PEG2000 concentration) allows precise control of particle size and improves uptake by immune cells like CD+ dendritic cells.
Active (ligand-mediated) targeting
Active targeting uses ligands, peptides, or antibodies conjugated to the LNP surface. One of the most effective tools for this is DSPE-PEG2000-maleimide, which serves as a linker to attach targeting molecules.
- Mannose-modified LNPs enhance uptake by dendritic cells for immunotherapy.
- Anti-CD4 and PECAM-1 antibodies guide LNPs to T cells or endothelial cells, enabling targeted delivery to the immune system or lungs.
Overcoming liver tropism
A known limitation of traditional LNPs is their hepatic tropism - a tendency to accumulate in the liver. While beneficial for liver-targeted therapies, it hinders delivery to other organs.
Emerging solutions include:
- Altering LNP surface charge and lipid composition
- Using "nanoprimers" to transiently block liver uptake
- Employing stealth strategies to prolong circulation time
These advances allow broader therapeutic reach—expanding tLNPs beyond hepatic applications.
Therapeutic applications of targeted LNPs
Cancer immunotherapy
LNPs are playing a major role in oncology, particularly for mRNA cancer vaccines. Many biotech companies are developing clinical-stage vaccines that stimulate the immune system against tumor antigens, offering a personalized approach to cancer treatment.
Pulmonary and CNS delivery
tLNPs are being explored for:
- Respiratory diseases (e.g., cystic fibrosis, influenza)
- Neurological disorders, by designing LNPs to cross the blood-brain barrier
Gene editing
Targeted LNPs are ideal carriers for CRISPR-Cas9 components, allowing gene editing therapies with tissue-specific precision—minimizing off-target effects and enhancing safety.
Conclusion: The Future of Targeted Lipid Nanoparticles
Targeted lipid nanoparticles represent a next-generation drug delivery platform. As research advances, their specificity, efficacy, and safety will only improve. Future innovations in ligand design, surface chemistry, and payload versatility will make tLNPs essential for personalized medicine, especially in oncology, immunotherapy, and genetic disease treatment.
At Avanti Research, we're committed to enabling precision in LNP development. From PEG-lipids to functionalized lipids like DSPE-PEG2000-maleimide, we provide the building blocks to take your tLNP formulations to the next level.
Revolutionize ligand-mediated targeting with DSPE-PEG2000-maleimide from Avanti Research—engineered for precision, designed for results.