Unlocking the Secrets of Lipid Formulation: Navigating Tm and Th for Optimal Processing and Troubleshooting Success
Formulating with lipids has many nuances, with perhaps temperature considerations being the most important processing aid. Individually, phase transition temperatures are provided as Tm and Th. Let’s discuss what these terms mean and how these characteristics impact processing, as well as how you may want to delve into temperature changes during formulation troubleshooting.
First, a few quick definitions. Tm is an abbreviation for the melt transition temperature, and with respect to lipids, this is the temperature at which the lipids will transition phases from the gel-ordered state with tightly packed fatty acid chains to the liquid crystalline state where the fatty acid chains are in a state of disorder and can be easily manipulated to form vesicles of smaller sizes (note: this is also the state you need to achieve for remote loading). Th is the abbreviation for the hexagonal transition temperature, which is the temperature some lipids will further transition into a hexagonal architecture. The hexagonal phase is not relevant to all lipids, and is most synonymous with the ethanolamine headgroup.
Many glycerophospholipids have published transition temperatures, and we include this information on our website for reference. Others that are not published can be determined experimentally through differential scanning calorimetry (DSC), which will yield a thermogram with endotherms to indicate the transition temperature of the particular lipid.
Some generalizations that can be helpful with respect to transition temperatures are below.
- Longer fatty acid chains of the same headgroup will have higher transition temperatures.
- Increasing amounts of double bonds are inversely related to transition temperature, resulting in decreased phase transition temperatures for molecules with more double bonds.
- Transition temperatures increase with headgroups PC<PS<PE<PA.
- PG and PC headgroups have almost the same transition temperatures related to the attached fatty acid chains.
When formulating with lipids, the transition temperature must be considered for processing, as the lipids need to be maintained in the disordered crystalline state to effectively form particles of particular sizes. A single-component lipid formulation will require hydration and processing above the transition temperature. When working with multi-component systems, we recommend working above the highest transition temperature in your system. For example, systems that include DSPC (18:0 PC), Cholesterol, and DSPE-PEG2000 should be hydrated and processed above 55°C because that is the transition temperature associated with DSPC, which is higher than both cholesterol and DSPE-PEG2000.
So, what happens if processing temperatures are below that of the lipid with the highest transition temperature? Your particles will not include all lipid components in the desired compositional ratios. In our example above, with a formulation composed of DSPC, cholesterol, and DSPE-PEG2000, if processing temperatures are 35°C instead of 60°C, the hydrated lipid blend will appear white, and a film may not suspend into the hydration buffer; extrusion membranes will easily be blocked with unhydrated lipids, homogenizer pressures can peak above working range due to large aggregates entering the chamber, and any resulting particles will not be evenly distributed with the components in the desired ratios.
What if an API, protein, or other substance cannot withstand such high temperatures or for sustained periods of time? Processing temperatures can be reduced by including different lipid species with lower transition temperature, or by including/increasing cholesterol as part of the formulation. In cases where formulation changes are not an option and working at extreme temperatures need to be avoided or avoided for long periods of time, we recommend doing two important tests to ensure formation of homogeneous particles: ensure molecularly bound lipid system mixtures, and perform DSC on the mixture to know the minimum working transition temperature of your blended system.
Molecularly bound lipid systems require mixing all lipid components together in a solvent system to achieve complete dissolution. Lipid solvent solutions that are well dissolved will be completely clear in clarity (while some color may be present due to inclusion of specific lipids). Techniques that may facilitate dissolution of lipid blends that are being pernickety include: adding heat, using a solvent system composed of multiple solvents, or dissolving each lipid individually and then mixing all solutions together. Standard dissolution solvents include chloroform, methanol, and ethanol. Solvent systems may include two or more of these solvents, in addition to water, hexane, isopropanol, ethyl acetate, etc. To ensure your lipid blend is homogeneous, a DSC thermogram of your dry lipid mixture will have a single endotherm; some thermograms may include broad peaks or shoulders, but a single peak indicates that the lipid blend has been thoroughly mixed and the peak of the endotherm is the transition temperature of the newly formed blend. During processing, stay above this transition temperature for effective particle preparation.
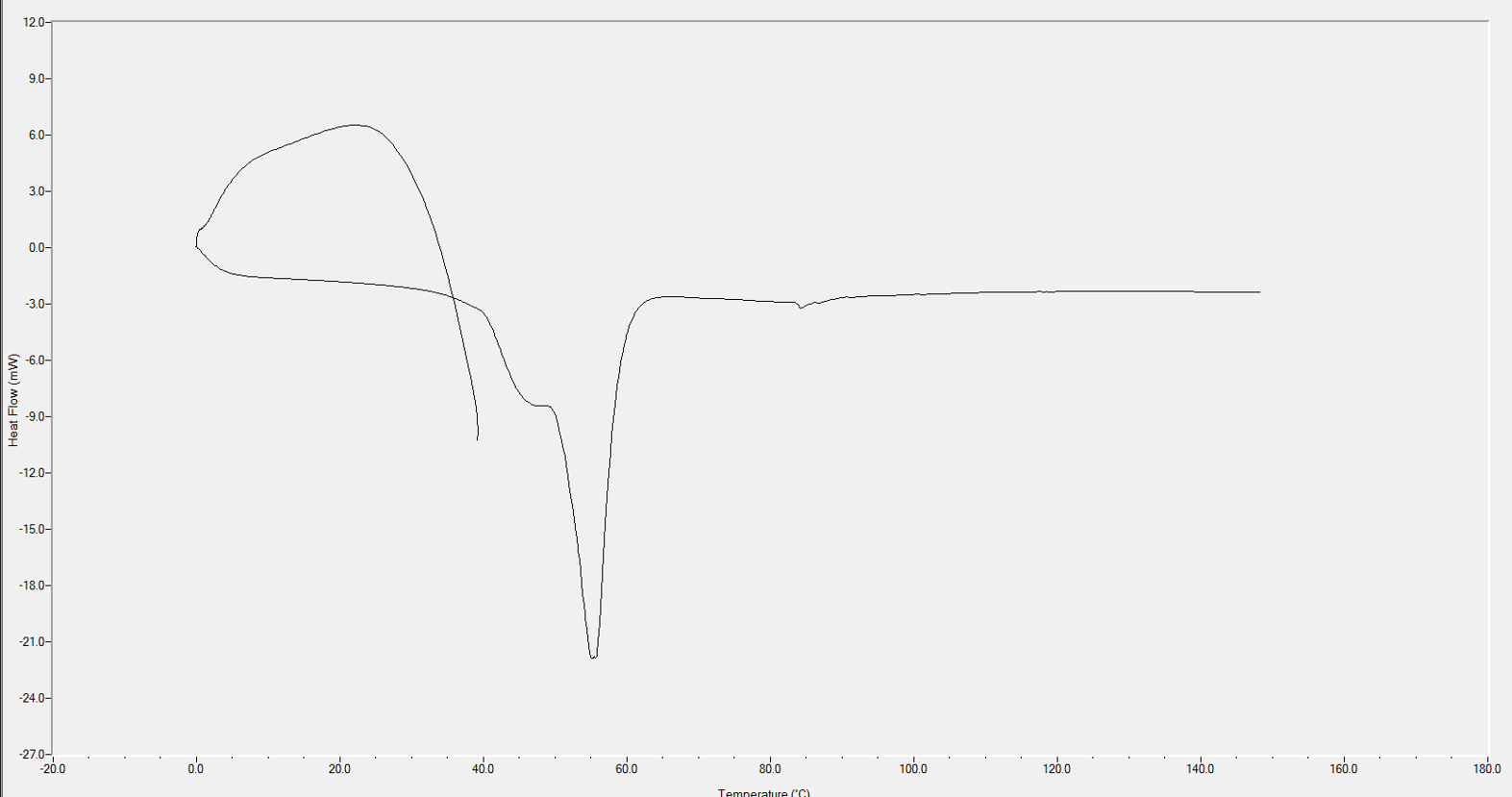
In conclusion, understanding and carefully considering phase transition temperatures are paramount when formulating lipid-based systems. The melt transition temperature (Tm) and hexagonal transition temperature (Th) play crucial roles in determining the lipid phases, impacting the effectiveness of particle formation and size control. As we navigate the complexities of lipid formulation, it becomes evident that temperature management is not merely a procedural detail but a key determinant in achieving the desired outcomes. By grasping the relationships between fatty acid chains, double bonds, and headgroups, formulators gain insights that guide them through the labyrinth of lipid behavior. Whether relying on published data or employing experimental techniques like differential scanning calorimetry, the pursuit of optimal processing temperatures is indispensable. It's not just about reaching the transition temperatures; it's about surpassing them for efficient particle preparation. As we delve into the nuances of lipid systems, we find that this knowledge not only aids in troubleshooting but serves as the compass guiding the creation of homogeneous particles with precise compositional ratios. In situations where high temperatures are impractical for certain components, molecularly bound lipid systems emerge as a strategic solution, offering a pathway to maintain homogeneity while avoiding excessive heat exposure. In the realm of lipid-based formulations, success hinges on a delicate dance with temperatures, ensuring that every lipid component contributes harmoniously to the final composition.
Click here for more information on phase transition temperatures for glycerophospholipids!